Research
MAQC Consortium 2011–2014 (read more)
FDA SEQC, Nature Biotechnology (read more)
Bioinformatics (read more)
FEBS Journal (read more)
Gerontology (read more)
Novel conserved repeats in sorting signals
There has been an increasing interest in single amino acid repeats ever since it was shown that these are the cause of a variety of diseases. Subsequently, such repeats have been implicated in a number of roles, including the facilitation of adaptive processes and in protein interaction networks.
In generic surveys, leucine repeats, which can be
toxic, surprisingly were among the most frequent. We
could show that repeats of leucine – but not of
other hydrophobic amino acids – are
over-represented in signal peptides that are cleaved
off the mature protein. This trend is most pronounced
in higher eukaryotes, particularly in mammals. In the
human proteome, although less than one-fifth of all
proteins have a signal peptide, approximately
two-thirds of all leucine repeats are located in these
transient regions.
 The substantial fraction of proteins affected by the
strong enrichment of repeats in these transient
segments also highlights the bias that they can
introduce for systematic analyses of protein
sequences.
The substantial fraction of proteins affected by the
strong enrichment of repeats in these transient
segments also highlights the bias that they can
introduce for systematic analyses of protein
sequences.
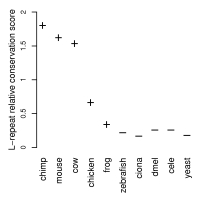
Moreover, in contrast to a general lack of conservation of repeats, these leucine repeats were found to be more conserved than the remaining signal peptide regions, indicating that they may have an as yet unknown functional role.
Reference
Łabaj PP, Leparc GG, Bardet AF, Kreil G, Kreil DP (2010) Single amino acid repeats in signal peptides, FEBS Journal 277, 3147. (read more | Supplement | preprint)
Return to group home or publications